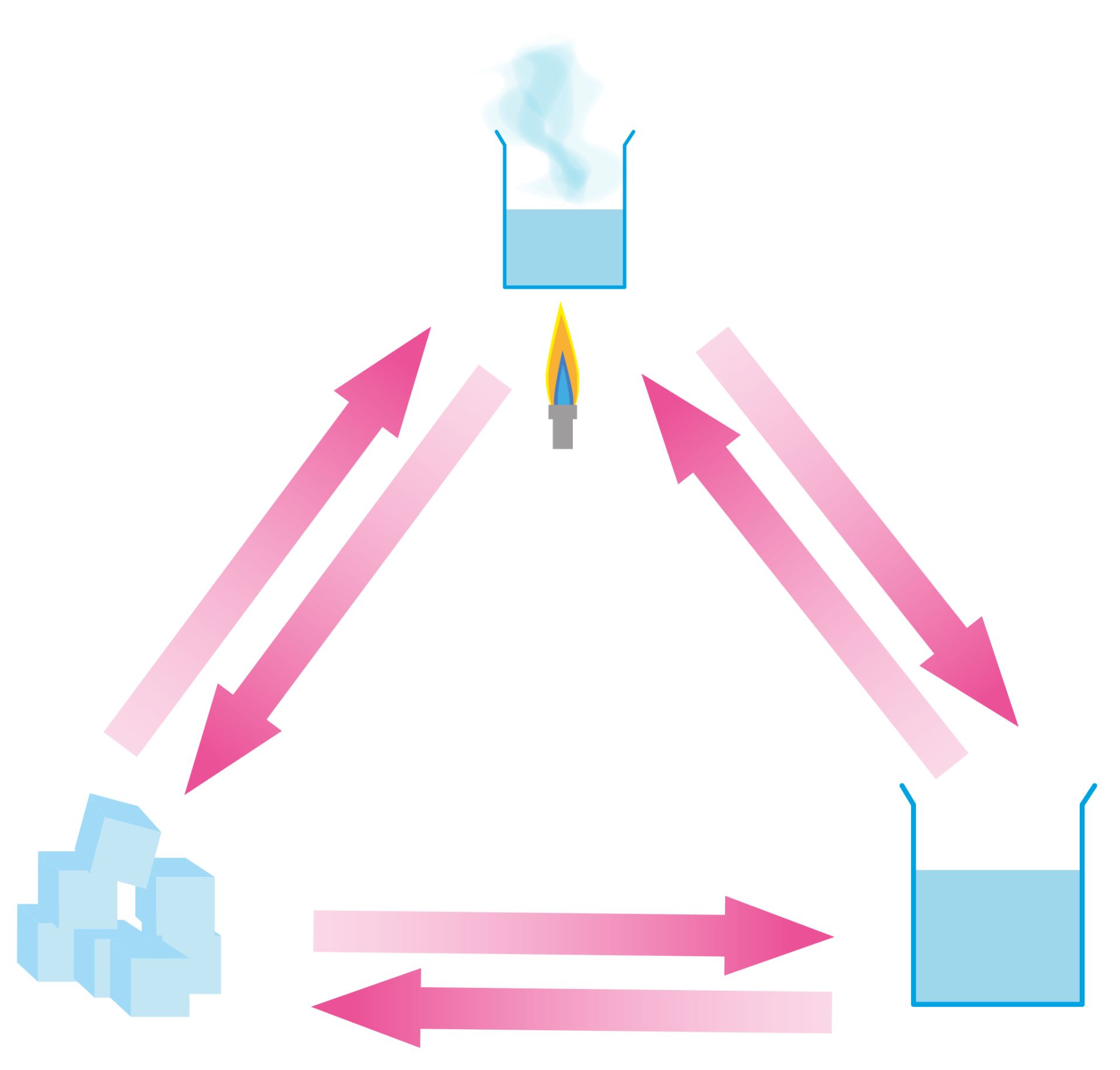
What Factors Cause Changes Between the Liquid and Gas State
Gas rarr liquid And thus steam water vapour condenses to liquid water. This causes the water vapor to change state and become tiny drops of liquid water.
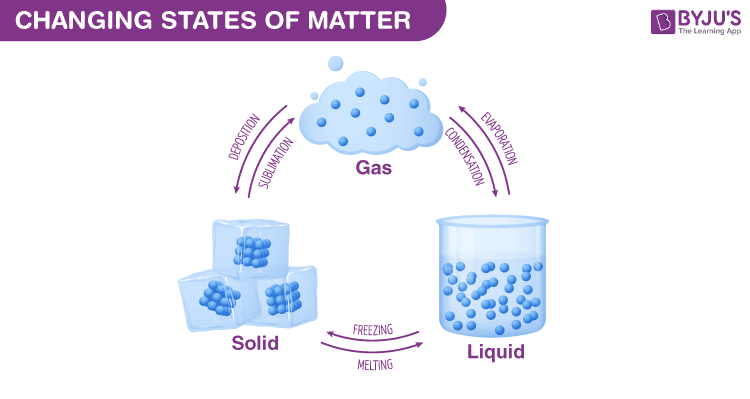
Changing States Of Matter For Kids Dk Find Out
Energy is transferred from the water vapor to the cup which cools the water vapor.
. A gas can be converted into a liquid by decreasing the pressure of a gas sample. Intermolecular forces are strongest in the solid state and weakest in the gaseous state for any given material. See full answer below.
What factors cause changes between the liquid and gas state. If you heat a liquid strongly enough eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. A gas can be converted into a liquid by increasing the pressure of a gas sample a gas can be converted into a liquid by cooling a liquid can be converted to a gas by heating.
Condensation describes the phase transition. For molecules of a liquid to evaporate they must be located near the surface be moving in the proper direction and have sufficient kinetic energy to overcome liquid-phase intermolecular forces. O A gas can be converted into a liquid by cooling ChA liquid can be converted to a gas by cooling D A liquid can be converted to a gas by heating O A gas can be converted into a liquid by heating.
Boiling is a phase transition from the liquid phase to the gas phase that occurs at or above the boiling temperature. The liquid and gas state. One of these factors is temperature.
In general solubility of a gas in water will decrease with increasing temperature. Several factors affect the solubility of gases. As a gas a substance does not have a fixed volume or shape.
The temperature rises until the water reaches the next change of state boiling. Liquids can vaporize into gases or freeze into solids. For example liquid water turns into steam when it is heated enough and it.
Substances can change state usually when they are heated or cooled. Find step-by-step Chemistry solutions and your answer to the following textbook question. If you have ever watched water boil you have witnessed heat as.
Solids can melt into liquids or sublime into gases. Other forms of energy besides thermal energy can change the state of matter. A liquid can be converted to a gas by cooling.
So if we increase the pressure of a gas that will cause the transit like if we reduce the pressure of the liquid thatLL make it easy for the boiling like those air generally the things that were talking about the factors that were talking about and that caused transitions. This happens as particles of liquid water gain enough energy to completely overcome the force of attraction between them and change to the gaseous state. For instance the steam that comes out of a hot teakettle making the whistle sing is water in the form of a gas.
Changes of state between liquid and gas. They rise to the surface and escape to the surroundings forming a gas. Often types of energy are absorbed by a material and change into thermal energy.
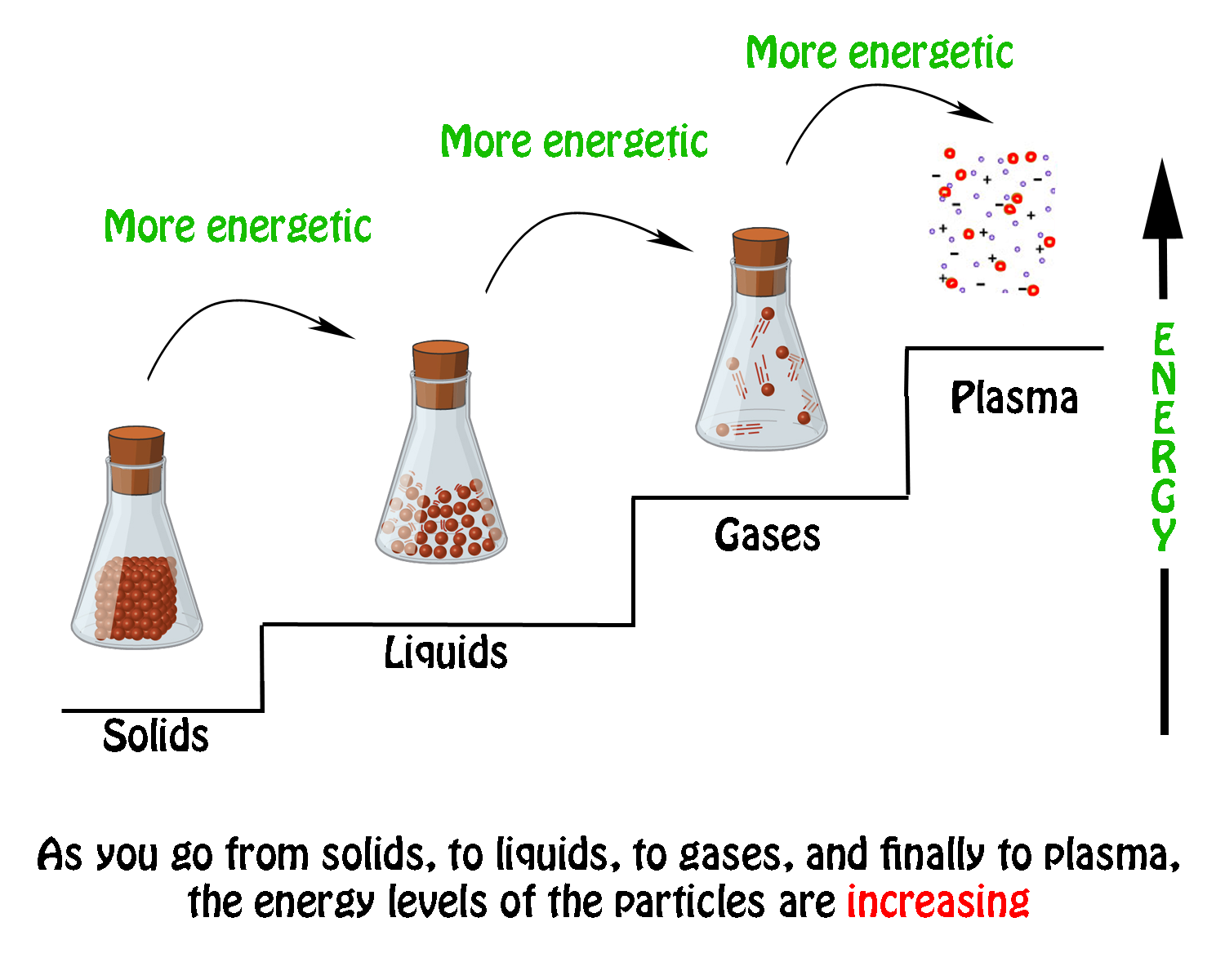
Gases can ionize into plasma condense into liquids or undergo deposition into solids. Solids form by deposition from gases or freezing of liquids. Gas expands to fill the shape and volume of its container.
H_2Og rarr H_2Ol Delta The process as written is exothermic and of course the condensation reaction can be used to drive a steam engine. When a liquid is heated it turns into a gas. Methane oxygen carbon monoxide nitrogen and helium all have different.
The process in which a liquid boils and changes to a gas is called vaporization. Heat and pressure together facilitate the change between the liquid and gas state. A gas can be converted into a liquid by increasing the pressure of a gas sample.
Energy from light can break chemical bonds to change a solid into a liquid. When the water vapor cools enough the attractions between the molecules bring them together. Liquids form by condensation of gases and melting of solids.
Colder water will be able to have more gas dissolved in it. In boiling bubbles of gas form throughout the liquid. The process by which a substance moves from the liquid state to the gaseous state is called boiling.
Changing state of matters. The intermolecular forces between water molecules decrease when a liquid turns into a gaseous liquid or steam. As the particles move faster and faster they begin to break the attractive forces between each other and move freely as steam a gas.
Um And in addition to heat transfer guiding the change between liquids and gases we also have to think about pressure. Heat causes substances to change their state because when heated the molecules within the. A gas can be converted into a.
The process of changing from a gas to a liquid is called condensation. For example adding electrical energy can ionize atoms and change a gas into plasma. A gas can be converted into a liquid by decreasing the pressure of a gas sample.
Gases form from the. In evaporation particles leave a liquid from its surface only. On constant vibration intermolecular forces decrease and particles start moving away from each other and change to gas.
The bubbles rise through the water and escape from the pot as steam. A gas can be converted into a liquid by increasing the pressure of a gas sample. Solubilities of Gases in Water.
What factors cause transitions between the solid and liquid state. A liquid can be converted to a gas by heating.

Phase Definition Facts Britannica

Changing States Of Matter Solid Liquid And Gas Phase Change
For The Three States Of Matter Solid Liquid And Gas There Are Six Possible Changes Of State Which Changes Of State Are Exothermic And Which Are Endothermic Socratic

Comments
Post a Comment